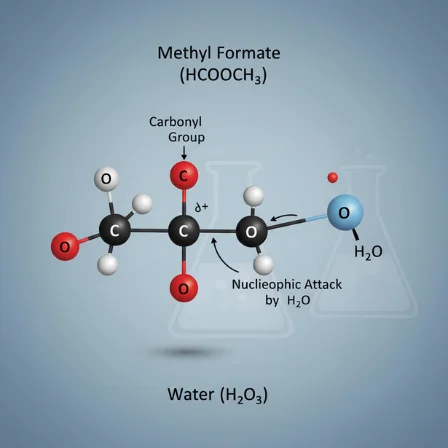
If you’ve ever come across the curious string of letters and numbers HCOOCH CH2 H2O in a textbook or research paper, you might have done a double-take. It doesn’t look like your standard chemical formula, and that’s because it isn’t one. This unconventional notation isn’t meant to represent a single, stable molecule you can bottle up. Instead, HCOOCH CH2 H2O is a fascinating piece of conceptual shorthand used by organic chemists to tell a story.
It encapsulates a fundamental and incredibly important reaction: the hydrolysis of an ester in water. More specifically, it’s a compact way of discussing the transformation of methyl formate into formic acid and methanol. Let’s pull this puzzle apart and see what makes this notation and the chemistry it represents so crucial, from the lab bench to large-scale industrial processes.
Breaking Down the HCOOCH CH2 H2O Notation
At first glance, HCOOCH CH2 H2O seems like a jumble. The key to decoding it is to stop thinking of it as one entity and start seeing it as a reaction snapshot.
The “HCOOCH” portion points directly to an ester, most commonly methyl formate (HCOOCH₃). Esters are known for their often-fruity smells and are central to everything from flavors to plastics. The “H₂O” is, plainly, water. The odd one out is the “CH₂” tucked in the middle. This isn’t a free methylene group floating around; it’s a symbolic placeholder. It represents the transformative step, the “moving part” of the reaction—the methyl fragment from the ester as it gets converted into methanol.
So, the full story HCOOCH CH2 H2O tells is this: Methyl formate + Water → Formic Acid + Methanol. In a proper chemical equation, it looks like this:
HCOOCH₃ + H₂O → HCOOH + CH₃OH
This hydrolysis reaction is a cornerstone of organic chemistry, and using this shorthand allows educators and researchers to quickly frame discussions about mechanism, reactivity, and the role of water.
The Core Players in the HCOOCH CH2 H2O System
To appreciate the reaction, we need to meet the main characters.
- The Ester (HCOOCH₃): Methyl formate has a structure that makes it ripe for reaction. It contains a carbonyl group (C=O), and the carbon atom in this group is electrophilic, meaning it’s somewhat electron-poor and hungry for nucleophiles. This makes it a perfect target for an attack by water.
- The Role of Water (H₂O): Here, water is far more than just a solvent. It acts as the nucleophile, attacking the ester. It also serves as a proton shuttle, facilitating the transfer of hydrogen atoms throughout the reaction, especially in the acid-catalyzed version. Its dual ability to donate and accept protons makes it uniquely powerful in driving this transformation.
- The CH₂ Concept: As mentioned, the methylene (CH₂) in the HCOOCH CH2 H2O notation is symbolic. In mechanistic discussions, it can help visualize the point of cleavage and the formation of the new alcohol. It reminds us that the carbon skeleton is being rearranged and broken apart.
The Chemical Mechanism Behind HCOOCH CH2 H2O
The hydrolysis of methyl formate can travel one of two primary pathways, each with its own steps and consequences. Understanding these mechanisms reveals the elegant dance of electrons and protons that the simple notation HCOOCH CH2 H2O implies.
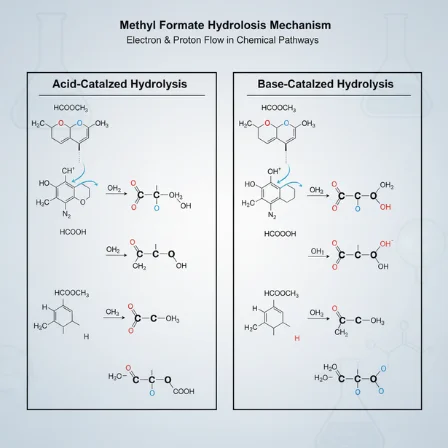
Acid-Catalyzed Hydrolysis
This route is reversible and is often sped up by an acid like sulfuric acid or hydrochloric acid.
-
Protonation: The carbonyl oxygen of the ester grabs a proton (H⁺) from the acidic solution. This gives the carbonyl carbon a stronger positive charge.
-
Nucleophilic Attack: A water molecule attacks this now highly electrophilic carbon.
-
Tetrahedral Intermediate: This forms a short-lived, four-centered intermediate.
-
Proton Transfer & Collapse: Internal proton shuffling occurs, turning one group into a good leaving group. The intermediate collapses, kicking off a molecule of methanol (which gets protonated briefly).
-
Deprotonation: Finally, a water molecule removes a proton from the nascent formic acid, yielding the final product and regenerating the acid catalyst.
Because it’s reversible, this process reaches an equilibrium. To get a good yield, chemists often use a large excess of water or continuously remove one of the products.
Base-Catalyzed Hydrolysis (Saponification)
This pathway, using a base like sodium hydroxide, is irreversible and goes to completion.
-
Nucleophilic Attack: A hydroxide ion (OH⁻), a much stronger nucleophile than water, directly attacks the carbonyl carbon of the ester.
-
Tetrahedral Intermediate Formation: Again, a four-centered intermediate forms.
-
Collapse: This intermediate collapses, expelling a methoxide ion (CH₃O⁻) and forming a formate ion (HCOO⁻).
-
Irreversible Step: The methoxide ion is a strong base. It immediately snatches a proton from a water molecule, producing methanol and regenerating a hydroxide ion.
The reaction is driven forward because the carboxylate product (formate ion) is very stable and has no tendency to revert to the ester under basic conditions.
Factors Influencing the HCOOCH CH2 H2O Reaction
The speed and efficiency of the process hinted at by HCOOCH CH2 H2O depend on several variables:
| Factor | Effect on Hydrolysis Rate | Reason |
|---|---|---|
| Temperature | Increases rate | Provides kinetic energy for more frequent and forceful molecular collisions. |
| Catalyst Strength | Increases rate | Stronger acids or bases lower the activation energy of the rate-determining step. |
| Water Concentration | Increases yield (in acid case) | Shifts equilibrium toward products (Le Chatelier’s principle). |
| Steric Hindrance | Decreases rate | Bulky groups near the carbonyl block the approach of the nucleophile. |
Industrial Applications of the HCOOCH CH2 H2O Process
The chemistry behind HCOOCH CH2 H2O is far from just academic. The hydrolysis of methyl formate is a workhorse reaction in the chemical industry.
- Formic Acid Production: This is a primary method for producing formic acid. This simple acid is a versatile chemical used as a preservative in livestock feed, a coagulant in rubber production, a descaling agent, and even in leather tanning and textile processing.
- Methanol Generation: The co-product methanol is a massive commodity chemical. It’s used as a solvent, a feedstock for making formaldehyde and acetic acid, and as a potential fuel and energy carrier in its own right.
The process’s relevance has grown with the push toward green chemistry. Hydrolysis reactions often use water as a benign reagent and can be optimized to run under milder conditions with less energy. Furthermore, there is exciting research into closing the carbon cycle by creating formate esters from captured CO₂, then hydrolyzing them. This could provide a sustainable pathway for carbon capture and utilization, making the simple reaction a potential player in addressing climate challenges.
Conclusion
The notation HCOOCH CH2 H2O is a brilliant example of how chemists use language and symbols to pack complex ideas into a compact form. It’s a gateway to understanding ester hydrolysis—a reaction that is mechanistic training for students, a tool for synthesists, and an industrial process with global importance.
From the subtle dance of protons in a flask to the large-scale production of essential chemicals, the story it tells bridges fundamental science and applied technology. The next time you see an unconventional formula, remember it might just be hiding a world of transformative chemistry.
If you’re fascinated by how simple molecular interactions power complex industries, consider exploring the principles of green chemistry or sustainable chemical engineering to see how reactions like these are being optimized for a better future.
Learn about MyGreenBucks
I’m Salman Khayam, founder and editor of this blog, with 10 years of experience in Travel, Lifestyle, and Culture. I share expert tips on Destinations, Hotels, Food, Fashion, Health, and more to help you explore and elevate your lifestyle.