Peptide Formulation Science: Innovation and Laboratory Basics for AOD-9604 and Acetic Acid 0.6%
In modern laboratories, peptide formulation science connects raw peptide powders to reliable test solutions. Researchers depend on carefully designed formulations to protect sensitive amino acid chains from damage. Good formulation does not chase trends or promises. Instead, it focuses on stability, consistency, and clear documentation inside controlled research environments. Every step exists to support accurate data rather than end-user applications.
Peptides can degrade through heat, light, pH shifts, or oxidation. Because of this, small preparation errors can undermine entire data sets. Well-planned peptide formulation science reduces that risk through standard methods and strict record keeping. This article explores key principles, peptide preparation methods, and two common tools. Those tools are AOD-9604 Peptide and acetic acid 0.6% in research chemistry workflows.
Why Peptide Formulation Science Matters for Data Quality
Strong peptide formulation science builds the foundation for trustworthy experimental results. If concentration, pH, or storage conditions drift, measurements can lose meaning. Two runs of the same assay may then show conflicting outcomes. The problem may not sit in biology at all. It may come from inconsistent handling or unstable solutions.
A robust formulation strategy offers several clear benefits for research teams:
- More consistent peptide concentration from vial to vial.
- Reduced risk of aggregation, precipitation, or oxidation in solution.
- Cleaner comparison between the new and historical study data.
- Fewer failed experiments due to avoidable handling issues.
- Stronger compliance with internal and external quality expectations.
When laboratories invest in peptide formulation science, they invest in repeatability. Each controlled step turns complex chemistry into dependable tools for in vitro protocols. Researchers then interpret trends with more confidence and less guesswork.
Core Peptide Preparation Methods: From Powder to Solution
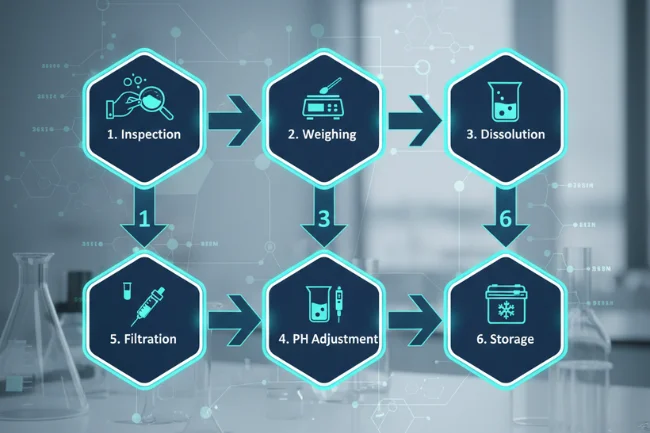
Most peptide preparation methods follow a clear sequence. This sequence runs from inspection to long-term storage. Each step builds on the previous one. Skipping any step creates hidden risk for later experiments. The following workflow outlines a practical, lab-friendly approach.
Step 1: Inspection, Identification, and Labeling
Start with a basic visual check of every vial. Confirm the label, batch number, and supplier documentation. Check the appearance of the powder for clumping or contamination signs. Record arrival dates and planned storage locations. Create secondary labels that highlight research-only use and hazard information.
Step 2: Accurate Weighing and Calculation
Next, move to weighing under controlled conditions. Use a calibrated analytical balance inside a low-draft environment. Let the balance stabilize before recording any values. Use anti-static tools and disposable weighing boats to avoid loss. Log the exact mass and calculate target concentrations for the final solution.
Step 3: Dissolution and Initial Mixing
Dissolution sits at the center of peptide formulation science. Choose a suitable solvent based on peptide properties and planned assays. Common options include sterile water or gentle buffered solutions. Add only a small volume at first and swirl the vial slowly. Allow time for the powder to fully wet before topping up to volume.
For some sequences, gentle warming or extended mixing may help. Always document any additional steps beyond the base protocol. Avoid vigorous shaking because foaming can trap air and harm delicate structures. A controlled, patient approach usually gives better clarity and stability. Consistency here protects every later measurement.
Step 4: pH Adjustment and Buffering
Once the peptide dissolves, check the solution pH. Many peptides show optimal behavior in narrow pH ranges. Adjust with compatible buffers or mild acids and bases. Introduce these modifiers slowly while monitoring with a calibrated meter. Record every adjustment so future batches match the same conditions.
Step 5: Filtration, Aliquoting, and Storage
When the solution meets clarity and pH targets, prepare for storage. Pass the solution through low protein-binding filters when protocols allow. This step removes particulates and supports downstream analytical work. Aliquot the filtered solution into labeled vials or microtubes. Store according to validated conditions, such as defined cold temperatures.
Limit freeze–thaw cycles by preparing smaller aliquots. Track each vial’s usage in a shared log. These practical peptide preparation methods help teams trace every sample. That traceability supports audits, troubleshooting, and long-term study review. It also aligns with good laboratory practice expectations.
AOD-9604 in Peptide Formulation Science
AOD-9604 offers a useful case study within peptide formulation science. Labs treat this fragment-based tool as a sensitive research material. They pay close attention to diluent choice, storage, and handling routines. Clear documentation connects each batch of solution to experimental results. This link supports later analysis and method refinement.
Teams that work with the AOD-9604 peptide usually define rigorous standards. They specify stock concentrations, acceptable appearance, and maximum storage times. They also record how many freeze–thaw cycles each aliquot experiences. With this detail, researchers can compare data across months or projects. Trends then reflect biology rather than uncontrolled preparation differences.
In practice, AOD-9604 encourages better habits in peptide formulation science. Its handling needs push teams toward more deliberate workflows. Those workflows then benefit many other peptide tools in the same lab. The result is a culture that values precision and traceability. That culture strengthens every project that touches peptide solutions.
Role of Acetic Acid 0.6% in Peptide Preparation Methods
Mild acids often support peptide dissolution and pH control. A dilute acetic acid solution sits among the most common options. At 0.6%, acetic acid offers manageable acidity with useful flexibility. Researchers integrate it into peptide formulation science for several reasons. It supports solubility while staying compatible with many analytical methods.
Technicians might use an acetic acid 0.6% compound to pre-wet stubborn powders. They may also include it in mobile phases for chromatographic analysis. In each case, the lab defines clear procedures and safety steps. Gloves, eye protection, and good ventilation remain non-negotiable requirements. All containers carry labels showing strength, date, and hazard class.
When teams combine acetic acid with specific peptide preparation methods, they test first. Small pilot batches reveal how the acid affects clarity, pH, and stability. Researchers then lock in conditions that support reliable data. They also document any limitations for certain assays or detection systems. This balanced approach keeps workflows safe, consistent, and reproducible.
Designing Robust Peptide Formulation Workflows
Effective peptide formulation science extends beyond a single vial. It covers the entire lifecycle of each research material. That lifecycle runs from ordering and receipt through disposal. Strong workflows treat every stage as part of one quality chain. The following practices help laboratories strengthen that chain.
Standard Operating Procedures and Checklists
Draft clear SOPs for key peptide preparation methods. Include detailed steps, required equipment, and acceptance criteria. Use checklists during preparation to reduce missed details. Review these documents regularly as new data and tools appear. Encourage staff feedback so procedures match real bench conditions.
Documentation, Tracking, and Version Control
Create batch records for every formulated solution. Log masses, solvents, pH values, filter types, and storage locations. Connect each batch to specific assay runs and analytical results. Digital systems often simplify this connection across teams. Version control also prevents confusion when protocols evolve.
Practical, Actionable Steps for Lab Teams
- Assign a formulation lead to oversee peptide-related workflows.
- Train new staff with hands-on demonstrations of each method.
- Audit storage units for correct labeling and segregation.
- Calibrate balances, meters, and temperature probes on fixed schedules.
- Review one recent project each quarter for formulation lessons.
These steps help teams capture the full value of peptide formulation science. They turn theory into practical routines that support every experiment. Researchers then spend less time troubleshooting and more time analyzing results. The lab gains efficiency, confidence, and stronger scientific outputs. That benefit touches both small discovery projects and large screening campaigns.
Future Directions and External Learning Resources in Peptide Formulation Science
Innovation in peptide formulation science continues to accelerate. Automation platforms now handle complex dilutions with high precision. Digital recipe systems guide technicians through each preparation step. Analytics tools highlight links between formulation choices and assay outcomes. Together, these advances reshape how labs design peptide workflows.
Researchers also learn from external technical communities and specialized sites. These sources discuss new solvents, packaging formats, and stability data. For a broader context, teams may consult resources such as specialist wellbeing and laboratory science hubs. Labs should always cross-check advice with internal validation, however. Local regulations, equipment, and assay needs still frame final decisions.
As tools evolve, one principle remains stable. Good peptide preparation methods always support clarity, consistency, and traceability. Those three pillars keep research chemistry grounded and dependable. They also help teams adapt safely to new instruments and materials. Innovation works best when strong fundamentals already exist.,
Disclaimer:
For Lab Use Only: This article discusses specialized Peptide Formulation Science for professional research and in vitro analysis exclusively. The content is not intended for human use or consumption and offers no medical or health advice.
I’m Salman Khayam, founder and editor of this blog, with 10 years of experience in Travel, Lifestyle, and Culture. I share expert tips on Destinations, Hotels, Food, Fashion, Health, and more to help you explore and elevate your lifestyle.